Infrared spectra and fragmentation dynamics of isotopologue-selective mixed-ligand complexes
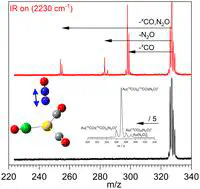 Image credit: RSC, Phys. Chem. Chem. Phys.
Image credit: RSC, Phys. Chem. Chem. Phys.Abstract
Isolated mixed-ligand complexes provide tractable model systems in which to study competitive and cooperative binding effects as well as controlled energy flow. Here, we report spectroscopic and isotopologue-selective infrared photofragmentation dynamics of mixed gas-phase Au(12/13CO)n(N2O)m+ complexes. The rich infrared action spectra, which are reproduced well using simulations of calculated lowest energy structures, clarify previous ambiguities in the assignment of vibrational bands, especially accidental coincidence of CO and N2O bands. The fragmentation dynamics exhibit the same unexpected behaviour as reported previously in which, once CO loss channels are energetically accessible, these dominate the fragmentation branching ratios, despite the much lower binding energy of N2O. We have investigated the dynamics computationally by considering anharmonic couplings between a relevant subset of normal modes involving both ligand stretch and intermolecular modes. Discrepancies between correlated and uncorrelated model fit to the ab initio potential energy curves are quantified using a Boltzmann sampled root mean squared deviation providing insight into efficiency of vibrational energy transfer between high frequency ligand stretches and the softer intermolecular modes which break during fragmentation.