Electronic structure of PH2− containing complexes as photoelectron spectroscopy candidates
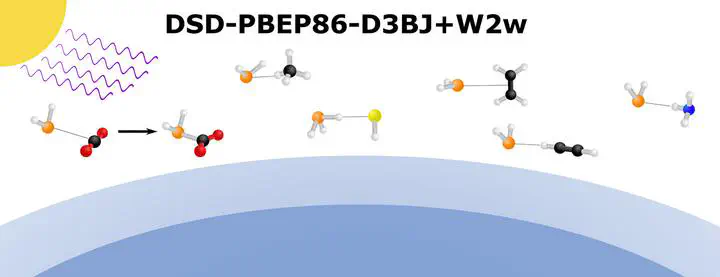 Image credit: Elsevier, Comput. Theor. Chem.
Image credit: Elsevier, Comput. Theor. Chem.Abstract
Phosphine is attracting interest due to the role it plays in extraterrestrial and interplanetary atmospheric environments. Phosphorus hydride anion and neutral van der Waals complexes with CH4, C2H4, HCCH, CO2, H2S and NH3 have been investigated as potential targets for photoelectron spectroscopy using a combination of DSD-PBEP86-D3BJ optimised geometries, W2w energies and Franck–Condon simulations. All anion complexes exhibit hydrogen-bonding interactions and while complexes with CH4 are weakly bound, showing minimal perturbation upon complexation. All other solvating molecules studied yield sufficient energetic or vibrational structural differences to be differentiable in photoelectron spectra. Most notably, PH2−$\cdots$CO2 undergoes barrierless nucleophilic addition to form the phosphino formate anion (PH2COO−) and is proposed as one such potential atmospheric sink.