 Image credit: ACS, J. Phys. Chem. A
Image credit: ACS, J. Phys. Chem. AAbstract
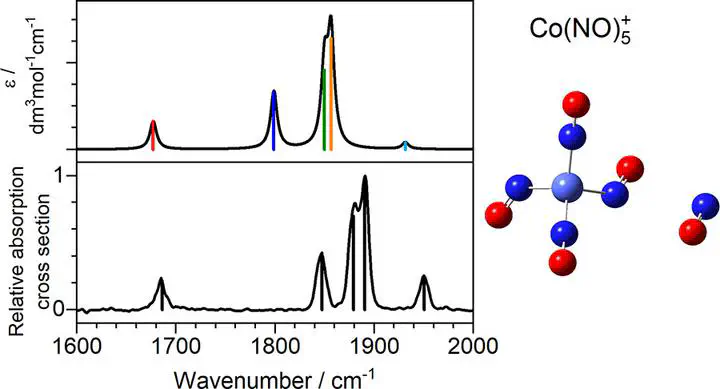
We present a combined experimental and quantum chemical study of gas-phase group 9 metal nitrosyl complexes, M(NO)n+ (M = Co, Rh, Ir). Experimental infrared photodissociation spectra of mass-selected ion-molecule complexes are presented in the region 1600 cm–1 to 2000 cm–1 which includes the NO stretch. These are interpreted by comparison with the simulated spectra of energetically low-lying structures calculated using density functional theory. A mix of linear and nonlinear ligand binding is observed, often within the same complex, and clear evidence of coordination shell closing is observed at n = 4 for Co(NO)n+ and Ir(NO)n+. Calculations of Rh(NO)n+ complexes suggest additional low-lying five-coordinate structures. In all cases, once a second coordination shell is occupied, new spectral features appear which are assigned to (NO)2 dimer moieties. Further evidence of such motifs comes from differences in the spectra recorded in the dissociation channels corresponding to single and double ligand loss.