Photoelectron Spectroscopy and High-Level Ab Initio Calculations of the Iodide–Methylperoxy Radical Complex
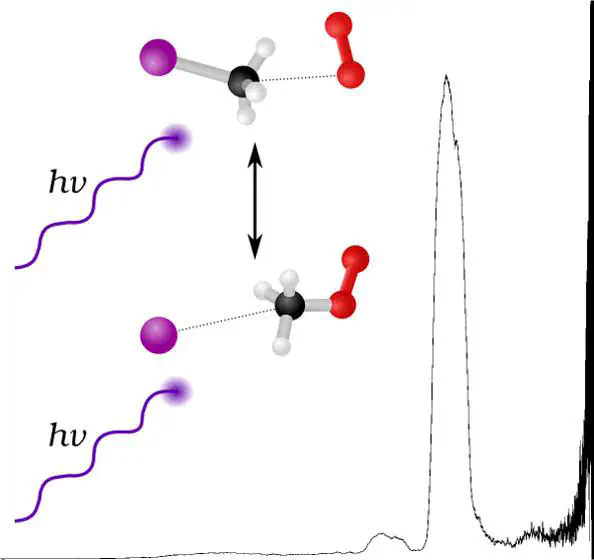 Image credit: ACS, J. Phys. Chem. A
Image credit: ACS, J. Phys. Chem. AAbstract
Anion photoelectron spectroscopy has been used to investigate the structure and dynamics of CH3OOI– van der Waals complexes. Peaks within the photoelectron spectrum are attributed to photodetachment to the perturbed 2P3/2 state of I$\cdots$CH3OO (3.46 eV) and the two 2P states of bare iodine. A broad feature at 1.7–2.4 eV is attributed to detachment to the excited singlet states from two O2–$\cdots$CH3I complexes. This represents the first anion photoelectron spectroscopy of a halide-bound methylperoxy radical species. Complex structures have been optimized using MP2/aug-cc-pVQZ with single-point energies at W1w theory for ground-state complexes and NEVPT2 for photodetachment to excited O2. Interactions are dominated by electrostatics, with the anion species interacting with the methyl pocket of the solvating molecule, suggesting conversion via an SN2 mechanism, and excess energy leading to complex dissociation within the timescale of mass spectrometry. The calculated W1w Gibbs energies suggest that while an electron transfer (ET) pathway to conversion is available, it is comparatively unfavored.