Halides and the carbon-carbon double bond: Interactions of ethylene with bromide and iodide
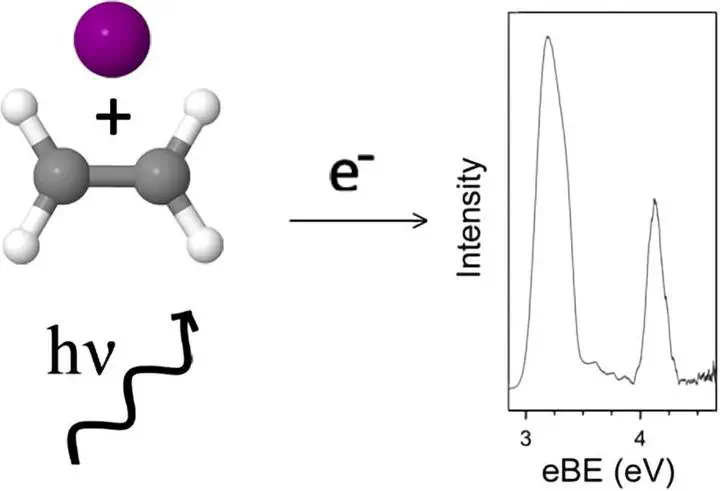 Image credit: Elsevier, Chem. Phys. Lett
Image credit: Elsevier, Chem. Phys. LettAbstract
A combined experimental and theoretical approach has been used to study the bromide-ethylene and iodide-ethylene gas-phase complexes. Experimental anion photoelectron spectra show the electron stabilisation of the complexes to be 0.19 eV and 0.14 eV associated with the bromide and iodide complexes respectively. High-level CCSD(T) calculations predict two minima with respect to each halide; the halide either appends linearly to a single hydrogen of the ethylene molecule or appends orthogonally to the carbon–carbon double bond. The two structural motifs are found to be close in energy, with the difference in dissociation energy associated with both halides determined to be approximately 1.3 kJ mol−1.
Type
Publication
Chemical Physics Letters, 793