 Image credit: Wiley, ChemPhysChem.
Image credit: Wiley, ChemPhysChem.Abstract
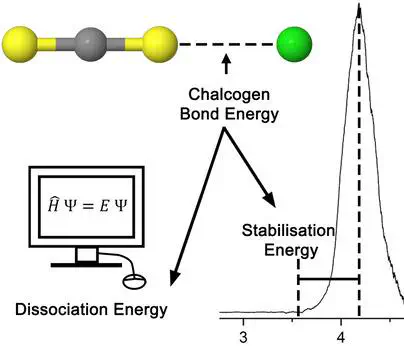
A combined experimental and theoretical approach has been used to study intermolecular chalcogen bonding. Specifically, the chalcogen bonding occurring between halide anions and CS2 molecules has been investigated using both anion photoelectron spectroscopy and high-level CCSD(T) calculations. The relative strength of the chalcogen bond has been determined computationally using the complex dissociation energies as well as experimentally using the electron stabilisation energies. The anion complexes featured dissociation energies on the order of 47 kJ/mol to 37 kJ/mol, decreasing with increasing halide size. Additionally, the corresponding neutral complexes have been examined computationally, and show three loosely-bound structural motifs and a molecular radical.