The bromide-bromomethyl radical dimer complex: Anion photoelectron spectroscopy and CCSD(T) calculations
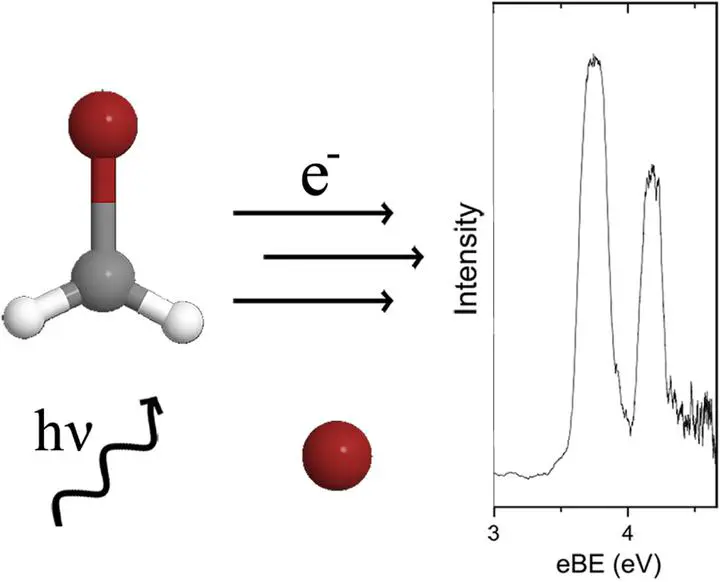 Image credit: Elsevier, Chem. Phys. Lett.
Image credit: Elsevier, Chem. Phys. Lett.Abstract
The gas phase anion photoelectron spectrum associated with the bromide-bromomethyl radical is presented. The stabilisation energy and electron binding energy, a property corresponding to the electron affinity of the neutral complex, are determined. Ab initio MP2 optimisations (with additional CCSD(T) energies) found two forms of the complex, a hydrogen bonded complex and a halogen bonded complex. The halogen bonded complex was found to exhibit C2v symmetry and the hydrogen bonded complex exhibited Cs symmetry. Comparison between the experimental data and the computational data allow conclusions to be drawn about the structure of the experimentally observed species.
Type
Publication
Chemical Physics Letters, 761