Characterisation of gas phase halide-acetone complexes with photoelectron spectroscopy and ab initio calculations
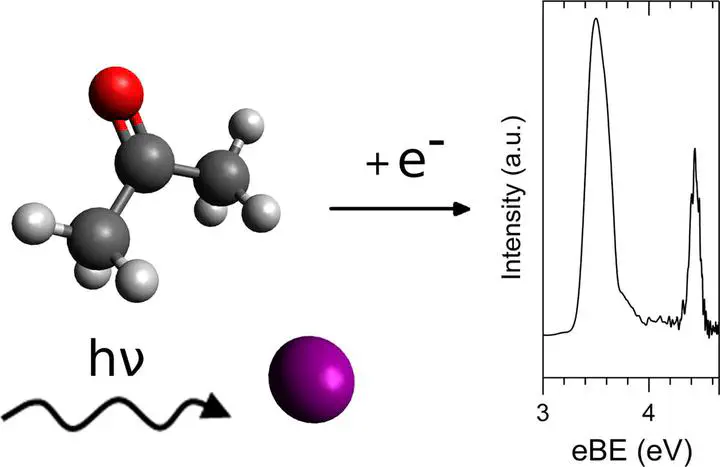 Image credit: Elsevier, J. Mol. Spec
Image credit: Elsevier, J. Mol. SpecAbstract
Anion photoelectron spectra of gas phase halide-acetone complexes (X−$\cdots$ CH3COCH3 where X = Cl, Br, I) are presented. Electron binding energies, corresponding to the electron affinities of the neutral species, are determined from experiment. Additionally, two ground state minima are predicted from MP2 calculations (with corresponding energies from CCSD(T) calculations) for each set of complexes: one Cs geometry for the anion complexes with the halide located midway between two hydrogens from each methyl group of acetone, and a C1 neutral structure with the halogen appended to the carbonyl oxygen.
Type
Publication
Journal of Molecular Spectroscopy, 364