 Image credit: Elsevier, Chem. Phys. Lett.
Image credit: Elsevier, Chem. Phys. Lett.Abstract
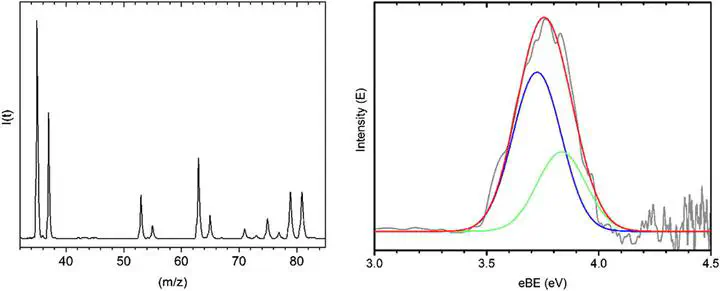
The gas phase anion photoelectron spectrum of the Cl− $\cdots$ N2 complex is presented, allowing determination of the electron binding energy, and is compared to CCSD(T) calculations. The calculations reveal three stationary points on the neutral complex surface; a linear C$\infty$v and two C2v symmetry geometries. For the anion complex, two geometries are predicted, similar to the C2v symmetry conformations determined for the neutral. Comparing both computational and experimental results with those from previous work we show trends between anion complex stability and electron stabilisation energy and also the neutral complex stability with respect to the polarisability of the halogen.
Type
Publication
Chemical Physics Letters, 654